On this page
- Learning about chronic inflammation for skin longevity
- Understanding the Roots of Inflammaging: How Subtle, chronic inflammation reshapes skin health over time
- Ectoin ® natural: A Threefold Defense Against Skin Inflammaging
- Proven by Science: The Clinical Impact of Ectoin® natural on Skin Health
- Rethinking Skin Longevity Through Cellular Resilience
Learning about chronic inflammation for skin longevity
Coined by Franceschi et al. in 2000, inflammaging describes the chronic, low-grade inflammation that increases with age and contributes to age-related diseases. The World Health Organization World Report on Aging and Health (2015) recognized the importance of strategies for healthy aging, and how skin health plays a central role in this conversation (although often overlooked).
Our skin is constantly exposed to both internal and external stressors – from UV radiation and pollution to microbial challenges. Over time, these stressors trigger a state of persistent, subclinical inflammation that accelerates visible signs of aging: wrinkles, uneven textures, slower regeneration and a weakened barrier.
At the molecular level, inflammaging involves a complex network of biological processes, including mitochondrial dysfunction, excessive reactive oxygen species (ROS), inflammasome activation, and the release of pro-inflammatory cytokines such as IL-1, IL-6, IL-8, and TNF-α. These pathways stimulate matrix metalloproteinases (MMPs) and the senescence-associated secretary phenotype (SASP), driving tissue degradation and impaired repair mechanisms.
Addressing these interconnected processes requires actives that go beyond surface-level effects. bitop Ectoin® natural has been shown to counteract several key mechanisms of skin inflammaging, helping to protect cells, stabilize membranes, and support long-term skin resilience.
In the sections ahead, we’ll take a closer look at how these actives work across these biological pathways and why they’re emerging as a cornerstone ingredient in skin longevity formulations.
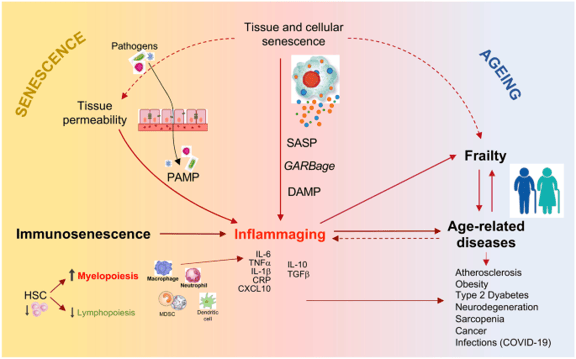
Figure 1 : Schematic network connecting senescence/immunosenescence, inflammaging, frailty, and age-related diseases.
Understanding the Roots of Inflammaging: How Subtle, Chronic Inflammation Reshapes Skin Health Over Time
Inflammaging doesn’t happen overnight, it’s a gradual shift in how our cells communicate and respond to stress. As we age, the body’s fine-tuned balance between repair and defense begins to blur, leading to a state of ongoing, low-level inflammation that silently drives visible and structural changes in the skin.
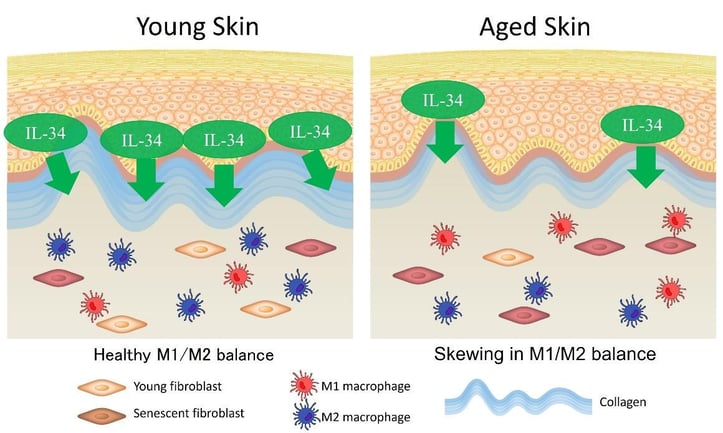
Figure 2: Imbalance of Macrophages leading to Inflammaging
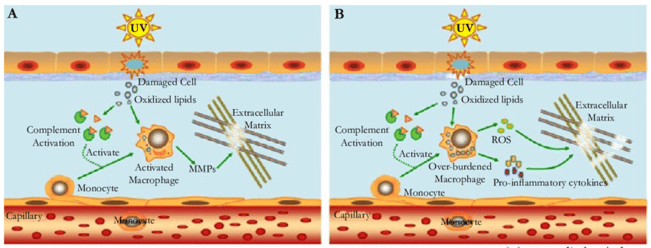
Figure 3: A model proposed to explain the mechanism of inflammaging in skin
The Five Stages of Inflammaging
Researchers have identified five distinct yet overlapping states of inflammaging: low-grade, controlled, asymptomatic, chronic, and systemic[2]. Together, these reflect how the body transitions from protective inflammation to one that becomes self-sustaining. These states arise from intricate interactions between cells and their surrounding microenvironment – a delicate balance of physiological and pathological signaling networks that, when disrupted, set the stage for chronic tissue stress and dysfunction.
Key Drivers of Inflammaging
Emerging research points to several interconnected biological mechanisms that drive inflammaging. While they operate through different pathways, all converge on the same outcome: cellular senescence, chronic inflammation, and progressive decline in tissue function.
Compromised Barrier Integrity
- Psychological, metabolic, and environmental stressors can weaken the body’s protective barriers – including the skin, gut lining, and blood-brain barrier. This allows microbial components such as lipopolysaccharides (LPS) to cross into deeper tissues, activating pattern recognition receptors (PRRs). The result is a continuous low-grade immune response that fuels inflammation and accelerates visible signs of aging.
Oxidative Stress
- Accumulation of reactive oxygen species (ROS) from mitochondrial dysfunction, environmental insults, and chronic metabolic activity leads to oxidative damage of proteins, lipids, and DNA. This activates NF-κB and other pro-inflammatory transcription factors, perpetuating a cycle of inflammation and cellular senescence.
Cytokine Dysregulation
- Cytokines, small signaling proteins secreted by immune and non-immune cells, are central to immune homeostasis. A balanced cytokine network supports tissue repair and regeneration, but with aging, cytokine secretion profiles shift toward a pro-inflammatory phenotype—a phenomenon referred to as "inflammaging cytokine signature." Key cytokines implicated include tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and interleukin-1β (IL-1β). Elevated systemic levels of these cytokines are considered biomarkers of inflammaging, correlating with frailty, immune dysregulation, sarcopenia, and increased morbidity and mortality [5,7,10].
Inflammatory Cascade Activation
- Continuous activation of the innate immune system—through Toll-like receptors (TLRs), complement pathways, and inflammasomes—leads to sustained production of chemokines, prostaglandins, and matrix-degrading enzymes. This cascade contributes to chronic tissue remodeling, immune cell infiltration, and feed-forward amplification of inflammation.
Looking Ahead: Cytokines at the Core
Among all the drivers of inflammaging, cytokines remain a focal point in current research. Their dual role, as both regulator and biomarker of inflammation, makes them essential for understanding and ultimately mitigating the effects of aging[5]. Aging studies show that a healthy balance of pro and anti-inflammatory cytokine secretion is associated with successful aging whereas dysregulation of this system results in inflammaging, poor aging phenotypes, and other aging related diseases [7]. Currently, levels of TNF-a, IL-6 and IL-1 can be used as inflammatory biomarkers that indicate frailty, an altered immune system, functional decline and mortality associated with inflammaging [10].
Ectoin ® natural: A Threefold Defense Against Skin Inflammaging
Ectoin® natural by bitop is a stress-protective cyclic amino acid known for its remarkable ability to stabilize and protect cells under extreme environmental conditions. In skincare, this translates into powerful protection for cellular structures and essential biomolecules against both internal and external stressors.
Its anti-inflammaging activity follows a threefold mode of action, addressing inflammation at every stage – from prevention and modulation to resolution and repair. By intervening early and supporting skin’s natural recovery processes, this active helps maintain homeostasis, resilience, and long-term skin health.
1. Prevention of Inflammation: Cellular Protection and Membrane Stabilization:
Ectoin® natural acts as a molecular shield, forming a protective water shell around cellular structures through its unique compatible solute mechanism. This hydration shell stabilizes cell membranes, lipids, and proteins, thereby:
- Preventing structural damage caused by environmental stressors such as UV radiation, pollution, and oxidative agents.
- Reducing reactive oxygen species (ROS) —a key trigger of inflammaging.
- Limiting the initial activation of pattern recognition receptors (PRRs) and subsequent NF-κB pathway stimulation, which prevents the transcription of pro-inflammatory mediators.
2. Treatment of Existing Inflammation: Cytokine and Pathway Modulation:
Ectoin® exerts a potent immunomodulatory effect, attenuating the inflammatory cascade by:
- Downregulating pro-inflammatory cytokines, including IL-1β, IL-6, and TNF-α, which are hallmarks of the inflammaging cytokine signature.
- Inhibiting NF-κB signaling, the central transcriptional regulator driving chronic inflammation and tissue degradation.
- Reducing oxidative mediators such as nitric oxide (NO) and prostaglandins, leading to decreased erythema, oedema, and inflammatory cell infiltration.
These combined effects interrupt the vicious cycle of chronic inflammation, mitigating tissue damage and slowing the progression of inflammaging.
3. Promotion of Repair: Barrier Restoration, Hydration, Skin Regeneration:
Beyond anti-inflammatory effects, Ectoin® enhances skin barrier integrity and supports regenerative processes by:
- Stimulating the synthesis of key barrier lipids (ceramides) and structural proteins (filaggrin).
- Decreasing transepidermal water loss (TEWL) and enhancing skin hydration.
- Supporting cellular regeneration and matrix remodelling, thereby reducing visible signs of aging such as roughness and fine lines.
Proven by Science: The Clinical Impact of Ectoin® natural on Skin Health
Long-Term Skin Barrier Repair an Anti-Inflammatory Efficacy (in -vivo)
In a double-blind, randomized, placebo-controlled clinical study the test products were a cream containing 1% Ectoin® natural, control (0.25% Hydrocortisone) “Ebenol® Cream“, placebo cream
1% Ectoin® natural reduced the TEWL by 24% and skin redness by 38% after only 7 days of treatment, due to its anti-inflammatory and repairing properties.
The efficacy of 1% Ectoin® natural was equivalent or even better than the results of the treatment with Ebenol®, proving its “Hydrocortisone-like” efficacy.
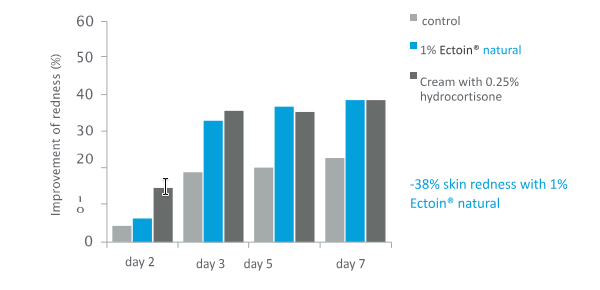
Figure 6: Average values of skin redness
Furthermore, a recent in vivo clinical study also proved the short-term anti-inflammatory efficacy of Ectoin® natural.
Short-Term Skin Barrier Repair and Anti-Inflammatory Efficacy (in-vivo)
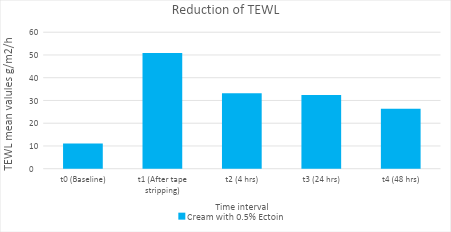
Single application of 0.5% Ectoin® natural is clinically proven to strengthen the skin barrier within 4 hours, with a reduction of TEWL and skin irritation by 60%.
0.5% Ectoin® natural also demonstrates long-term skin barrier and skin-soothing efficacy upto 48 hours (single application) with a reduction of TEWL by 72%.
Downregulation of cytokines, interleukin (IL)-1α, IL-6, IL-8, and TNF-α (in-vivo)
The efficacy of Ectoin® in downregulating cytokines, interleukins and TNF- α has been proven with two clinical trials. The first clinical trial was conducted on patients suffering from chronic lung inflammation and the second clinical trial was conducted on patients suffering from Chemotherapy-induced Oral Mucositis.
Both these clinical trials have demonstrated that Ectoin® down-regulates the expression of key inflammatory mediators, particularly:
- Interleukin-1 alpha (IL-1α): reduced by 30%
- Interleukin-6 (IL-6): reduced by 50%
- Interleukin-8 (IL-8): reduced by 45%
- Tumor Necrosis Factor alpha (TNF-α): reduced by 40%
Ectoin® effectively modulates inflammatory cytokines and interleukins (TNF-α, IL-1β, IL-6, IL-8), thereby showing excellent efficacy and tolerability for clinical use.
Rethinking Skin Longevity Through Cellular Resilience
In the evolving field of skin longevity, Ectoin® natural stands out as a clinically proven bioactive that tackles inflammaging at its roots. By addressing cytokine overexpression, oxidative stress, and impaired barrier function, it restores balance where ageing begins – at the cellular level.
Clinical studies show significant downregulation in key pro-inflammatory cytokines, IL-1α (↓30%), IL-6 (↓50%), IL-8 (↓45%), and TNF-α (↓40%), effectively interrupting the cycle of chronic low-grade inflammation that accelerates skin ageing. In both short and long-term in vivo studies, Ectoin® improved hydration, reduced erythema, and enhanced integrity. All while delivering efficacy comparable to hydrocortisone, but with higher safety and tolerability.
At the molecular level, Ectoin® inhibit NF-kB activation, modulates MAPK signaling, and mitigates ROS accumulation, protecting keratinocytes from premature senescence. Its threefold mechanism – prevention, treat and repair – supports cytokine homeostasis, reinforces the skin barrier, and promotes long-term regeneration.
Together, these effects establish Ectoin® natural as a next-generation bioactive for maintaining skin health, resilience and longevity. It bridges the gap between cosmetic innovation and therapeutic science.
Interested in exploring Ectoin® natural for your formulations? Request your sample today on Covalo, and experience how this molecule can power your next innovation.
References
- Krutmann J, et al. The role of extremolytes in skin protection: Molecular mechanisms and clinical evidence. J Dermatol Sci. 2020;98(3):123–131.
- Jantschitsch C, et al. Ectoine reduces cytokine expression in human keratinocytes exposed to environmental stress. Exp Dermatol. 2018;27(6):625–632.
- Kennedy, B. K. et al. Geroscience: linking aging to chronic disease. Cell 159, 709–713 (2014).
- Vierkotter A, Krutmann J. Environmental stress and skin aging: Protective role of compatible solutes. Dermatoendocrinol. 2012;4(3):264–270.
- S. Giunta, “Is inflammaging an auto[innate]immunity subclinical syndrome?” Immunity and Ageing, vol. 3, article 12, 2006
- C. Nathan and A. Ding, “Nonresolving inflammation,” Cell, vol. 140, no. 6, pp. 871–882, 2010.
- Tauber, A. I. Timeline: Metchnikoff and the phagocytosis theory. Nat. Rev. Mol. Cell Biol. 4, 897–901 (2003).
- Gregor, M. F. & Hotamisligil, G. S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 29, 415–445 (2011).
- Hotamisligil, G. S. & Erbay, E. Nutrient sensing and inflammation in metabolic diseases. Nat. Rev. Immunol. 8, 923–934 (2008).
- Hotamisligil, G. S. Inflammation, metaflammation and immunometabolic disorders. Nature 542, 177–185 (2017).